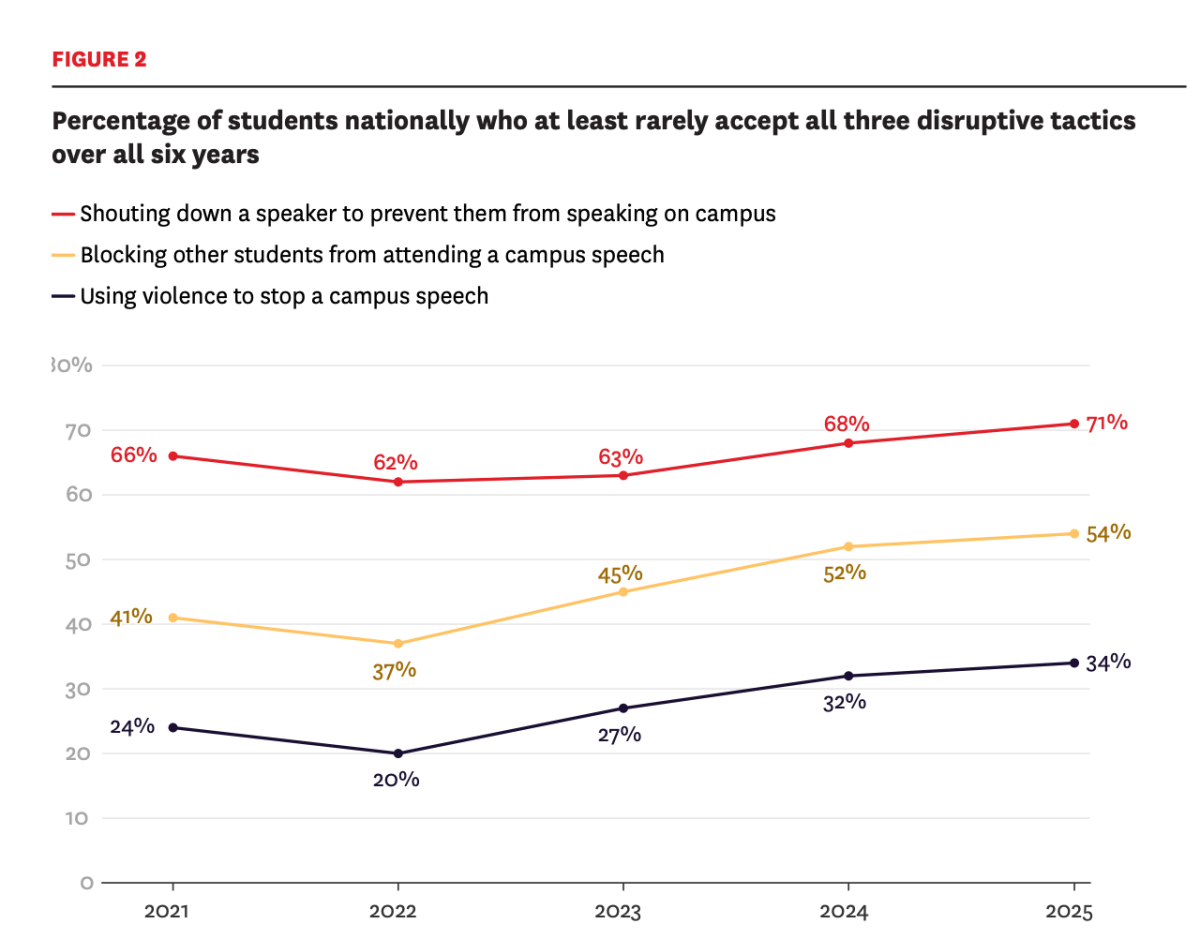
Blood banks implementing FDA’s lifting of ‘blood ban’ for gay, bisexual men
The shifting of the guidelines from the Food and Drug Administration comes after advocates presented research to the FDA highlighting the scientific irrelevance of the policy barring blood donations on the basis of whether or not it is a man who has had sex with man.
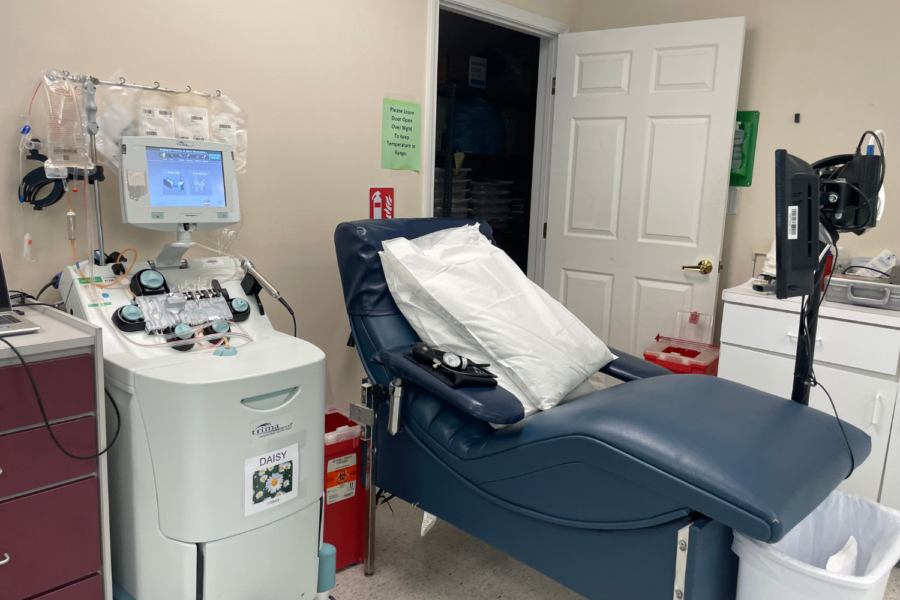
The Middleburgh Heights location for the blood donation center Vitalant. Vitalant worked with the FDA on the ADVANCE study, assesing which questions in an individual risk assesment would be effective in assesing HIV risk.
Later this year, local blood donation centers will implement the Food and Drug Administration’s updated guidelines made on May 11, which eases the blood donation restrictions for men who have sex with men, a policy long pointed out by advocacy groups as “discriminatory.”
Two blood donation centers local to the Berea area, the American Red Cross, with locations in Parma and Elyria, and Vitalant, with a location in Middleburg Heights, will be using the updated individual risk assessments in the months to come, or the forms which assess blood donor’s eligibility. The new forms will remove any gendered language, such as asking about whether the potential donor is a man who has had sex with another man and will instead ask whether the individual has had anal sex in the last three months or multiple sexual partners.
Kirstin Lane, the communications manager for Vitalant, said that Vitalant is proud to be taking part in the recent updates for donor eligibility.
“We worked very, very closely with the FDA on what we call the Advance Project [The ADVANCE Study], which was a study that really brought to the FDA’s attention the need to look more closely at their policies for blood donations that needed to be updated,” Lane said.
The ADVANCE Study, standing for Assessing Donor Variability And New Concepts in Eligibility, was an 1800-person study started in 2021 which assessed the scientific relevance of the FDA’s policy regarding blood donations from gay and bisexual men. The study drew blood from participants to test for HIV and asked them different questions to assess which questions of the assessment would be effective in testing for HIV risk factors.
This policy preventing gay and bisexual men from donating blood, originated from 1983 during the human immunodeficiency virus crisis when the FDA placed a permanent ban to avoid the transmission of HIV via blood transfusions.
In 1983, there was no reliable way to screen blood for HIV, but according to the CDC, blood donations were routinely screened for HIV-1 successfully by 1985 and HIV-2 by 1992. Since then, there have been routine screenings of all donated blood which is able to detect HIV reliably.
The ADVANCE Study was presented at the FDA’s Blood Products Advisory Committee, which included professionals throughout the United States who assessed the relevance of the policy. Pride and Plasma, a group led by 2023 University of Cincinnati graduate of nursing and political science, Cole Williams, was the only group present at the FDA’s Blood Products Advisory Committee. Pride and Plasma presented the FDA with the Advance Study, a petition with over 1,000 signatures, concerns from blood centers regarding the nationwide blood shortage and global trends regarding their policies.
“The science of today is not the science of the 1980s and hasn’t been for a long long time,” Williams said. “If [the FDA] had acted a little bit quicker, then we would have had this policy removed.”
Williams said that a significant aspect of this rule is that it removes any gendered language which makes the blood donation process easier for transgender women and nonbinary donors. While the FDA revised its rules in 2018 to ask blood donation centers to allow their donors to self-identify their gender instead of using their assigned sex at birth, there were reported incidents of non-enforcement.
“The policy [which barred individuals from blood donation on the basis of sexual orientation] was applied to trans women and non-binary donors incredibly frequently by any blood facilities that were not gender-affirming,” Williams said. “The removal of all gendered language and most recent updates that they just put out, it’s going to have a significant impact on removing barriers for those donors.”
At a blood drive on campus hosted by BW’s chapter of Circle K International through the American Red Cross, BW’s LGBTQ+ student group, Allies, was one source of pushback on the policy which barred blood donations based on sexual orientation, as they themselves petitioned for signatures outside of the centers. Now, the Allies’ co-presidents, Jena Parks and Randy Forshed, said that they plan to promote the lifting of the ban in the upcoming academic year.
“I’m sure we are going to promote at the blood drives themselves, as we have in the past with our petitions,” Parks said. “And I definitely want to push for social media posts specifically around those Circle K events, just spreading information, trying to get it out there that these individuals can now give blood.”
Williams said that while the current policy change is reflective of the current scientific research, there still has been pushback from some progressive groups who still view the policy as discriminatory because of the anal sex provision and the provision that defers individuals if they are taking HIV prevention medication such as PrEP. Forshed said that because of these provisions, given that there were more medical advancements, it would be nice to see the policy move further.
“I settle with it on the basis that there is change that we could come up with some more technological advancement to increase inclusivity,” Forshed said.
Pride and Plasma is now focusing its efforts on allowing men who have sex with other men to donate their tissues. This policy has been around since 1994 and is being called into question as “discriminatory.” Pride and Plasma is collecting a petition on tissue donation here: https://tr.ee/81ry5D0yYQ
The Exponent is looking for financial contributions to support our staff and our newsroom in producing high-quality, well-reported and accurate journalism. Thank you for taking the time to consider supporting our student journalists.